2025 Volume 73 Issue 1 Pages 39-45
2025 Volume 73 Issue 1 Pages 39-45
In the present study, the stability of a supersaturated solution of indomethacin (IM) was evaluated in hydrophobically modified hydroxypropylmethylcellulose (HM-HPMC) solutions, with and without parent cyclodextrins (CDs). A highly supersaturated state of IM was maintained in the HM-HPMC solution and was further stabilized by the addition of α-CD and β-CD. Notably, the highest level of supersaturation was achieved in HM-HPMC/α-CD solution, which maintained a high concentration of IM for up to 120 h. IM concentrations in these solutions exceeded the amorphous solubility, indicating that phase separation had occurred. To explore this phase separation, Nile Red, a fluorescent probe sensitive to hydrophobic environments, was added to the supersaturated solutions. A higher fluorescence intensity was observed in the HM-HPMC/α-CD solution compared with the HM-HPMC solution, indicating a significant formation of colloidal amorphous aggregates in the supersaturated solution. Cryogenic transmission electron microscopy (Cryo TEM) analysis confirmed the presence of these aggregates, which appeared irregularly shaped. These findings suggest that the combination of HM-HPMC and α-CD effectively stabilized the colloidal amorphous aggregates in the IM supersaturated solution. The addition of α-CD facilitated the dissociation of HM-HPMC into smaller particles, increasing the number of hydrophobic stearyl moieties available for interactions with amorphous IM aggregates, thereby enhancing the stability of the supersaturated state. The combination of HM-HPMC and α-CD offers a promising approach to improving the oral bioavailability of drugs with poor water solubility.
The majority of new drug candidates in drug development exhibit poor aqueous solubility, which often results in low oral bioavailability.1,2) Given that many patients prefer orally administered formulations, improving the aqueous solubility of drugs remains a critical challenge in drug development. To address this issue, various formulation strategies employ polymer carriers or additives, including self-emulsifying systems,3,4) nanocrystals,5) cyclodextrin (CD) complexation,6) and amorphous solid dispersions (ASDs).7,8) Among these, ASDs are of particular interest because the amorphous drug is dispersed in a hydrophilic polymer matrix, leading to the formation of a supersaturated solution during dissolution. The amorphous form, being in a higher energy state, generates a supersaturated concentration much higher than that of the crystalline form.9) However, the amorphous form is thermodynamically unstable and tends to crystallize into the more stable form during dissolution, leading to a reduction in drug concentration in solution over time. When the amorphous drug is prepared as an ASD, crystallization can be suppressed by the polymer, allowing ASD to maintain several-fold higher solubility (amorphous solubility) compared to the crystalline form.10–12) Recently, it has been reported that when the apparent drug concentration exceeds the amorphous solubility, which represents the maximum free drug concentration achievable in solution, phase separation can occur in aqueous media.13–16) If this phase separation occurs at a temperature above the drug’s glass transition temperature (Tg), it is referred to as liquid–liquid phase separation (LLPS). In contrast, if phase separation occurs below the Tg, it is known as glass–liquid phase separation (GLPS). The drug-rich phase generated via LLPS or GLPS possesses a high surface area and exists in a prewetted state, which enables it to act as a reservoir that rapidly supplies the drug to the supersaturated solution, thereby improving bioavailability.13)
Recently, various hydrophobically modified polymers, consisting of a water-soluble polymer with hydrophobic residues along the backbone or at the chain ends, have been developed for medical applications, including drug delivery.17–19) Hydrophobically modified hydroxypropylmethylcellulose (HM-HPMC) is a thickener that contains hydrophobic stearyl moieties in its polymer backbone,20–23) contributing to the formation of three-dimensional networks through intra- and intermolecular associations. Previous studies have shown that the addition of CDs modifies the association of HM-HPMC by encapsulating the stearyl moieties.24,25) In the preparation of ASD, polymers such as HPMC, poly(vinylpyrrolidone) (PVP), and their derivatives are frequently used to stabilize supersaturated drug solutions.7) However, to the best of our knowledge, the effect of HM-HPMC on the stability of supersaturated solutions has not been comprehensively evaluated, and no studies have examined the influence of HM-HPMC in the presence of CDs. CDs alter the association state of HM-HPMC in solution, increasing the number of stearyl moieties that may contribute to stabilizing drug supersaturated solutions. In this study, we investigated the effects of HM-HPMC and CDs on the stability of indomethacin (IM) supersaturated solutions. IM, with a relatively low Tg (wet Tg ≈ 29°C),26) was selected as a model drug due to its poor water solubility. In the presence of HM-HPMC and α-CD, a high supersaturated concentration of IM was achieved, which was characterized from the viewpoint of phase separation. Additionally, the stabilization mechanism of the supersaturated state of IM in HM-HPMC/α-CD solutions was explored in this study.
HM-HPMC (commercially known as Sangelose 60L) was obtained from the Daido Chemical Co., Ltd. (Osaka, Japan). The 60L HM-HPMC (M.W. 400000) contains a C-18 fatty acid group at the hydroxypropyl ends and has a degree of substitution (DS) of 0.3–0.6 mol%. The DS values for the hydroxypropoxy and methoxy groups in 60L HM-HPMC were 7.0–11.0 mol% and 27.0–30.0 mol%, respectively. α-, β-, and γ-CDs were obtained from Nihon Syokuhin Kako Co. (Tokyo, Japan). IM (form γ crystal) and Nile Red were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). The chemical structures of IM, HM-HPMC, and Nile Red are shown in Supplementary Fig. S1. All other reagents used in this study were of analytical grade and Milli-Q water was used throughout the study.
Solubility of IM in HM-HPMC and CDs SolutionsThe equilibrium solubility of IM was assessed in JP first fluid (pH 1.2) in the presence and absence of 0.1% (w/v) HM-HPMC and 0.1% (w/v) CDs. An excess amount of IM (5 mg) was added to 1.0 mL of these solutions, followed by shaking at 1200 rpm for 24 h at 37°C. To obtain the supernatant, the samples were subjected to ultracentrifugation at 100000 rpm for 10 min while maintaining the temperature at 37°C. The concentration of IM in the supernatant was analyzed using an HPLC system (JASCO, Tokyo, Japan). Separation was performed on an ODS-AM column (250 × 4.6 mm, 5 μm; YMC Co., Ltd., Tokyo, Japan) with a 20 × 4.0 mm, 5 μm ODS-A precolumn at 40°C. The mobile phase consisted of 0.1% phosphoric acid solution and acetonitrile (45 : 55). IM was detected at 262 nm with a flow rate of 1.2 mL/min after appropriate dilution of the samples with the HPLC mobile phase. The degradation of IM was negligible under the experimental conditions.
Stability of IM Supersaturated Solution in the Presence of HM-HPMC and CDsThe stability of the IM supersaturated solution was assessed by monitoring the apparent concentration of IM in the supersaturated state over time. The IM supersaturated solution was prepared by adjusting the pH of the IM solution in the presence or absence of HM-HPMC and CDs at 37°C. Specifically, 1.0 M HCl was added to 20 mL of a 2.0 mM IM solution, which had been prepared using 0.1 M phosphate buffer (pH 8.0), to lower the pH to 1.2. At predetermined intervals, 1.0 mL aliquots of the sample were collected and filtered through a fine filter F-72 (Toyama Sangyo Co., Ltd., Osaka, Japan) which effectively removes particles sized 2–5 μm with an efficiency of 62.1% and those sized 5–10 μm with an efficiency of 95.7%. The concentration of IM in the filtrate was determined by HPLC under the conditions described above.
Characterization of Amorphous IM Aggregates in the Supersaturated State Characterization by Fluorescence ProbePhase separation of IM in the supersaturated solution was confirmed using Nile Red, a fluorescent probe, that is sensitive to the hydrophobic environments.13) Ten microliters of a 200 μg/mL Nile Red methanol solution was added to 990 μL of the IM supersaturated solution, which had been prepared by adjusting the pH to 1.2. Fluorescence data were acquired using an infinite 200 pro microplate reader (TECAN, Switzerland) with the excitation wavelength set at 520 nm and the emission wavelength at 610 nm. The gain value was set to 133, and the temperature was maintained at 37°C. Fluorescence data were also collected from the IM solution prior to the addition of HCl, to subtract the fluorescence intensity observed in the HM-HPMC solution.
Cryogenic Transmission Electron Microscopy (Cryo TEM) MeasurementCryo TEM analysis was carried out using a JEM-2100F (JEOL Resonance Inc.) at an accelerating voltage of 120 kV. The film side of the microgrid adhesion grid (Cu200, Nisshin EM Co., Ltd., Tokyo, Japan) was hydrophilized for 60 s using HDT-400 (JEOL Resonance Inc.). A 2 μL aliquot of each sample solution was applied to the hydrophilized grid, and after removing the excess solution with filter paper, the cryo TEM samples were rapidly frozen in liquid ethane, which had been cooled approximately –170°C using liquid nitrogen. The samples were loaded onto a Gatan 626 cryo holder (Gatan Inc., CA, U.S.A.) under liquid nitrogen, and TEM measurements were conducted with the temperature maintained below –170°C.
Particle Size Measurement of HM-HPMCThe particle size of the HM-HPMC was determined using dynamic light scattering (DLS) with an ELSZ-1000 (Otsuka Electronics Co., Ltd., Tokyo, Japan). Measurements were conducted on a 0.1% (w/v) HM-HPMC solution, both in the presence and absence of CDs, with the temperature maintained at 37°C.
Interaction between IM and HM-HPMCThe interaction between IM and HM-HPMC in phosphate buffer (pH 8.0) was investigated using Nile Red as a fluorescent probe. Ten microliters of Nile Red methanol solution (200 μg/mL) was added to the 990 μL of a 0.1% (w/v) HM-HPMC solution, with or without 2 mM IM. Fluorescence data were acquired under the conditions described above, while the gain value was set to 167. Fluorescence data were also collected in the presence of 0.1% (w/v) α-CD.
First, the solubility of IM in HM-HPMC and CD solutions was investigated to define the supersaturated state of IM. The concentration of HM-HPMC was set to 0.1% (w/v) to maintain a low viscosity throughout the study. The solubility of IM in pH 1.2 phosphate buffer was 2.8 × 10–3 mM, while it slightly increased to 5.1 × 10–3 mM in the HM-HPMC solution (Fig. 1). This increase is probably due to interactions between the hydrophobic IM and the hydrophobic moieties of HM-HPMC, which solubilize IM in the solution. CDs also slightly increased the solubility of IM at this concentration, consistent with the stability constant of IM (Table 1, Supplementary Fig. S2). When both HM-HPMC and CD were added to the solution, the solubility of IM was almost the same as in the CD-only solution for both α-CD and β-CD. This is because the hydrophobic moieties of HM-HPMC are encapsulated by these CDs, preventing interactions between IM and HM-HPMC, as described in the following section. On the other hand, the solubility of IM increased when HM-HPMC was added to the γ-CD solution, reflecting the combined solubilizing effects of both HM-HPMC and γ-CD. This is probably because γ-CD, with its large cavity size, hardly interacts with the hydrophobic moieties of HM-HPMC.

Each value represents the mean ± standard error (S.E.) of 3 experiments. The concentrations of HM-HPMC and CDs were 0.1% (w/v). HM-HPMC: hydrophobically modified hydroxypropylmethylcellulose; CD: cyclodextrin.
 |
The stability of the IM supersaturated solution was evaluated by monitoring the concentration of IM in its supersaturated state, which was prepared by pH shifting of the solution. IM concentration rapidly decreased over time in the IM-only solution after the addition of HCl, eventually reaching a level close to the crystalline solubility of IM (Fig. 2a, white circle). A similar decrease in IM concentration was observed in the CD solutions, with the concentration reaching near the solubility of IM in the presence of CD. In contrast, a high concentration of IM was maintained in the HM-HPMC solution for 24 h (Fig. 2c, yellow circle). The IM concentration increased with the addition of CDs, in the order of α-CD > β-CD > γ-CD. These IM concentrations were higher than the amorphous solubility of IM (20 μg/mL, 5.6 × 10–2 mM) at pH 1.2, which was determined by the UV extinction experiments13) (Supplementary Fig. S3), and this value is lower than the previously reported amorphous solubility of 36 μg/mL at pH 2.2.27) The level of supersaturation (Figs. 2b and 2d) was calculated by dividing the apparent IM concentration in Figs. 2a and 2c by the solubility of IM in each solution (Fig. 1). As shown in Fig. 2d, in the presence of both HM-HPMC and α-CD, the highest supersaturated state of IM was observed for 24 h, with the IM concentration being 140–190 times higher than the solubility of IM in this solution. Changes in apparent IM concentration were monitored at various α-CD concentrations (Fig. 3), which demonstrated the highest supersaturation among the CDs. The IM concentration increased with increasing α-CD concentrations, but no further increase was observed beyond 0.1% (w/v) α-CD, while the highest level of supersaturation was achieved by the addition of 0.1% (w/v) α-CD (Fig. 3b). Since the solubility of IM increased with increasing α-CD concentration, the supersaturation level decreased at 0.5% (w/v) and 1.0% (w/v) α-CD, despite the same apparent IM concentration being observed in these solutions. The long-term stability of the IM supersaturated solution was evaluated in the presence of 0.1% (w/v) α-CD (Supplementary Fig. S4). The HM-HPMC/α-CD solution maintained a high concentration of IM above its amorphous solubility for 120 h, demonstrating a significant stabilization effect.

Each point represents the mean ± S.E. of 3–6 experiments. The concentrations of HM-HPMC and CDs were 0.1% (w/v). The dashed line indicates the amorphous solubility of IM. The level of supersaturation in (b) and (d) was calculated by dividing the apparent concentration of IM by its solubility in each solution, as determined in Fig. 1. IM: indomethacin; HM-HPMC: hydrophobically modified hydroxypropylmethylcellulose; CDs: cyclodextrins.

Each point represents the mean ± S.E. of 3–6 experiments. The level of supersaturation in (b) was calculated by dividing the apparent concentration of IM by its solubility in each solution. The dashed line indicates the amorphous solubility of IM. IM: indomethacin; S.E.: standard error; HM-HPMC: hydrophobically modified hydroxypropylmethylcellulose; CD: cyclodextrin.
In the presence of HM-HPMC, a high supersaturation level of IM was observed, which further increased with the addition of α-CD. The apparent concentration of IM in these solutions exceeded its amorphous solubility, suggesting that phase separation of IM occurred. To investigate this phase separation, Nile Red, a fluorescent probe sensitive to hydrophobic environments, was added to the supersaturated solutions. When IM in the supersaturated state forms hydrophobic colloidal amorphous aggregates, the fluorescence intensity of Nile Red increases in proportion to the amount of these aggregates.13) As shown in Fig. 4, the highest fluorescence intensity was detected in the HM-HPMC/α-CD solution, followed by HM-HPMC/β-CD, HM-HPMC/γ-CD, and the HM-HPMC-only solution. This trend aligns with the supersaturation levels in these solutions, demonstrating that colloidal amorphous aggregates formed in the HM-HPMC solution and that the amount of these aggregates increased further with the addition of α-CD. Phase separation occurred at 37°C, a temperature above the Tg of IM, suggesting that IM exists as a supercooled liquid. The amorphous aggregates formed in these solutions were evaluated using Cryo TEM (Fig. 5). Nano-sized aggregates smaller than 100 nm were observed in the supersaturated solutions and were well dispersed in the HM-HPMC/α-CD solution. Generally, amorphous drug aggregates in a supercooled liquid state (LLPS) exhibit a spherical shape,28) while the observed nanoaggregates were irregularly shaped. This irregularity is likely due to interactions between HM-HPMC and the surfaces of the amorphous aggregates, which altered their shape. It is also possible that the amorphous aggregates were in a glassy state, which may have caused their irregular shape. Hydrophobic HM-HPMC is likely to be incorporated into the IM nanodroplets, potentially inhibiting water access to the nanodroplets and altering the wet-Tg of IM. Further investigation is needed to clarify the cause of the irregular shape of the amorphous aggregates in the HM-HPMC solution. These results suggest that the combination of HM-HPMC and α-CD effectively stabilized the amorphous aggregates in the IM supersaturated solution.

Each value represents the mean ± S.E. of 3–4 experiments. IM: indomethacin; S.E.: standard error.

IM: indomethacin; TEM: transmission electron microscopy.
Hydrophobically modified polymers form associations through inter- or intramolecular hydrophobic interactions in water. CDs modify these polymer–polymer associations by forming inclusion complexes with the hydrophobic moieties on the polymer.29–32) It is probable that CD alters the association of HM-HPMC in water, thereby contributing to the stabilization of the supersaturated state of IM. To investigate the association state of HM-HPMC, the particle size of HM-HPMC was measured in the presence and absence of CDs (Fig. 6). HM-HPMC exhibits a particle size of 1100 nm at 37°C with 0.1% (w/v) in water, and this size decreased with the addition of α-CD and β-CD. Specifically, the particle size decreased to 360 nm with the addition of α-CD. Due to its small cavity size, α-CD readily forms inclusion complexes with alkyl chains, such as the stearyl group, whereas γ-CD, with its larger cavity size, has limited interactions with these groups.24) α-CD disrupted the associations of HM-HPMC more effectively than other CDs, resulting in a decrease in particle size. It can be estimated that viscosity and viscoelasticity would also change if the polymer associations were altered. However, no changes were observed in these parameters with the addition of CDs (Supplementary Fig. S5), indicating that the effects of viscosity and viscoelasticity of HM-HPMC on the stabilization of the supersaturated state of IM were negligible.

Each value represents the mean ± S.E. of 3 experiments. HM-HPMC: hydrophobically modified hydroxypropylmethylcellulose; CDs: cyclodextrins.
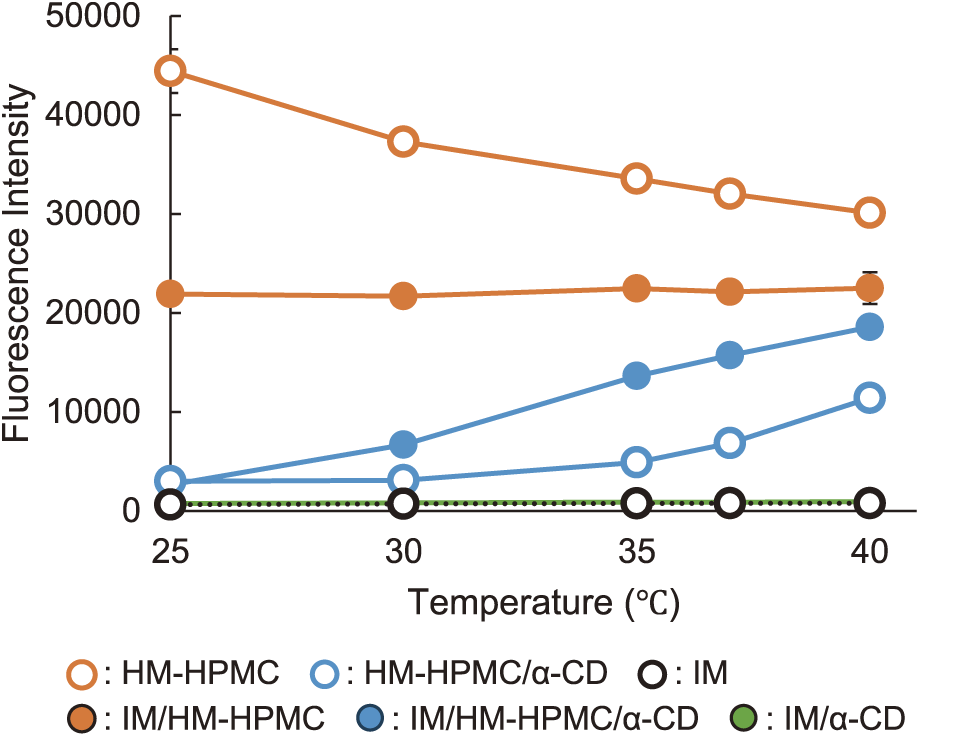
To estimate the stabilization mechanism of the supersaturated state of IM in the HM-HPMC/α-CD solution, interactions between IM and HM-HPMC were evaluated using Nile Red (Fig. 7). The fluorescence of Nile Red was measured in HM-HPMC solutions in the presence and absence of IM, which was not in a supersaturated state. A high fluorescence intensity was observed in the HM-HPMC solution due to the presence of hydrophobic stearyl moieties on the polymer. The fluorescence decreased with the addition of IM, indicating that IM competes with Nile Red for interactions with the hydrophobic stearyl moieties. In the HM-HPMC/α-CD solution, the fluorescence intensity markedly dropped where α-CD forms inclusion complexes that inhibit interactions between Nile Red and HM-HPMC. This low intensity increased with the addition of IM and was temperature dependent. The fluorescence intensity remained negligible in the α-CD solution alone, suggesting that IM competes with both α-CD and Nile Red for interactions with the hydrophobic stearyl moieties. These findings clearly indicate that the stearyl moieties on HM-HPMC contribute significantly to the interaction with IM, stabilizing its supersaturated state, with α-CD further enhancing this effect.
Each point represents the mean ± S.E. of 3 experiments. The concentrations of HM-HPMC and CDs were 0.1% (w/v), and the concentration of IM was 2 mM. IM: indomethacin; HM-HPMC: hydrophobically modified hydroxypropylmethylcellulose; CDs: cyclodextrins.
The stabilization mechanism of the supersaturated state of IM in the HM-HPMC/α-CD solution is summarized in Fig. 8. In the presence of HM-HPMC, a supersaturated solution of IM forms, with IM existing as colloidal amorphous aggregates. HM-HPMC likely inhibits the crystallization of IM from these aggregates, possibly due to interactions with its stearyl moieties. However, the number of stearyl moieties available for such interactions is limited because HM-HPMC tends to self-associate in water. With the addition of α-CD, HM-HPMC dissociates into smaller particles, increasing the number of stearyl moieties available for interactions with amorphous aggregates, thereby stabilizing the supersaturated state more effectively than HM-HPMC. Further investigations into the interactions among HM-HPMC, α-CD, and the amorphous aggregates are needed to fully clarify the stabilization mechanism. This will be reported in a separate study focusing on drugs with a low Tg, which exhibit high molecular mobility even within nanodroplets,33,34) in contrast to IM. Additionally, the equilibrium state among HM-HPMC, α-CD, and IM at the amorphous solubility level may also play a role in inhibiting IM crystallization.

IM: indomethacin; HM-HPMC: hydrophobically modified hydroxypropylmethylcellulose; CD: cyclodextrin.
A highly supersaturated solution of IM was obtained in HM-HPMC/α-CD solution, with IM existing as colloidal amorphous aggregates. α-CD modifies the association state of HM-HPMC by forming inclusion complexes with its stearyl moieties, transforming HM-HPMC into a more favorable form to stabilize IM amorphous aggregates. Such a high supersaturated state is expected to be achieved during dissolution from ASD. ASD prepared with HM-HPMC and α-CD may thus be a promising approach to improving the oral bioavailability of drugs with poor water solubility.
The authors declare no conflict of interest.
This article contains supplementary materials.